Katelin works with a dimethylmethylene blue (DMMB) assay in the lab to measure the amount of sulfated glycominoglycans in a sample of biomaterial.
Honors biomedical engineering student Katelin Cherry began working with mentor Dr. Jeffrey Wolchok last spring to create a biomaterial from skeletal cells that would ideally represent the complex components of skeletal muscle. A biomaterial of this type could be used for the regenerative treatment of damaged muscle tissues and Katelin plans to present her research at the United Kingdom’s Society for Biomaterials Conference this summer in London.
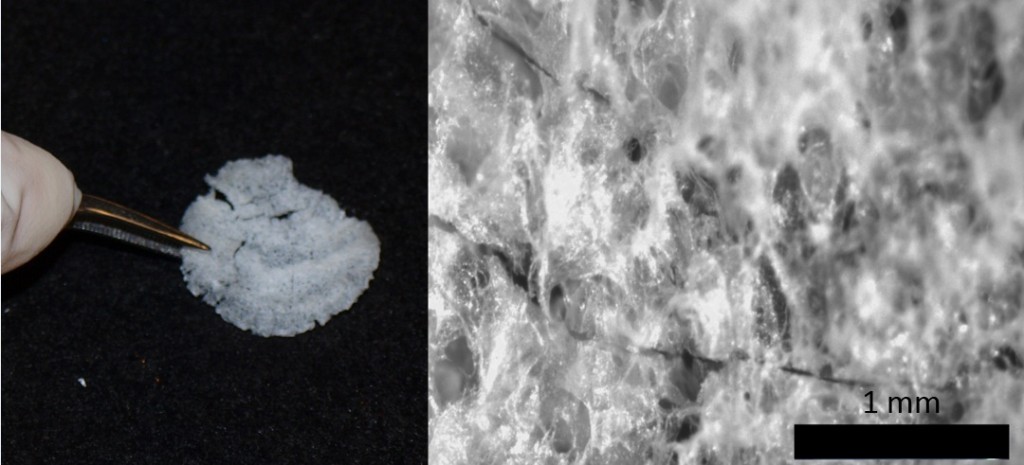
In the spring 2012 semester, I began my undergraduate research on engineering a skeletal cell derived biomaterial. Specifically, I was in interested in using this approach to create a material intended for the repair of damaged muscle tissue. The specific goal of my project was to use the sacrificial scaffolding approach with human muscle cells and determine the characteristics of the material that is produced. These characteristics were compared to published values for native human muscle. In the ideal situation, the material collected would be representative of the complex components of the skeletal muscle.
In the early spring semester, I began seeding foams with skeletal cells then I cultured the cells for 3 weeks. Afterwards, I removed the sacrificial plastic foam so only the biomaterial was remaining. Most importantly, for this research, I wanted to compare the collagen and glycoaminoglycan content of the biomaterial to real skeletal muscle. Collagen is a main component of the extracellular matrix of tissue. (Extracellular matrix is synthesized by the resident cells of each tissue and provides a support medium for blood or lymphatic vessels and nerves.) I planned to use both a hydroxyproline assay to determine the collagen content and dimethylmethylene blue (DMMB) assay to quantify the amount of sulfated glycoaminoglycans (another component of the extracellular matrix). Therefore, I began developing a standard curve for both of these assays, and I had some complications such as what concentrations to use and the technique of these processes.
In the fall semester, I was able to work out the problems concerning the standard curve and concentrations for the assays. I obtained values for the collagen content of both the muscle tissue and engineered muscle tissue. However, I was unsuccessful in obtaining values for the sGAG content of the engineered tissue. When performing this test, the samples are expected to obtain an absorbance reading similar to the standard curve created. The standard curve had absorbance readings of 0.3-.55 and varied in color from dark blue to purple. The engineered tissue had readings ranging from .7-1.0 and created a tealish color. Professor Jeffrey Wolchok and I tried to determine if there was another factor creating this strange result. We thought it might have been the buffer solution or the samples weren’t properly digested. We were unsuccessful in finding a solution to the problem however and we feel that the issues with the dimethylmethylene assay will have to be addressed in a future study.
Dr. Jeffrey Wolchok, an assistant professor in Biomedical Engineering, was my mentor for this project. He has a strong background in tissue engineering and has published papers on creating a cell derived extracellular matrix. His knowledge in this field, as well as in the process of conducting research, was very helpful to me while I was completing my project. He showed me how to seed foams using a sacrificial foam technique, how to culture cells and the importance of sterility, and how to conduct proper animal dissections. Also, he and I worked together to create a hydroxyproline assay and dimethylmethylene asssay for quantifying collagen within the tissue samples. Though we were unsuccessful in creating the dimethymethylene assay, we made good progress on that assay and in the future, the problems should be figured out. His lab is conducting many experiments within the field of tissue engineering and my research on growing skeletal cells has provided useful information for his own research.
This research project has helped me develop my skills as a researcher and has given me a taste of the scientific research process. Also, the general lab experience I have gained through this project has been very beneficial. I’ve taken classes that included lab sessions where I was required to perform cell culturing tasks and work in a laboratory, but I learned much more by conducting my own honors research project. I wasn’t performing tasks to obtain a good grade and learn basic concepts such as in a class; instead, I had a goal for my project and learned the importance of proper technique to achieve good results. I enjoyed the freedom that doing research offers. Though there were many days of frustration and a couple of dead ends, I felt accomplished when I was finally able to produce good results. I really enjoyed the process and I am now considering a career in research. I’ve always been a very curious individual, and this research gave me the opportunity to seek answers to my questions.
I plan on traveling to the United Kingdom’s Society for Biomaterials Conference this summer in London. At this conference, I will present my own research while learning more about regenerative medicine and the advancements that are being made in this field. As a whole, I am very thankful for my undergraduate research experience and the Honors College Research Grant which provided the necessary funding to conduct my research.